GTM Strategy in Regulated Industries
During a consulting engagement with a pharmaceutical company, I worked closely with Jennifer, their VP of Marketing, who was navigating the complex launch of a new therapeutic device. Jennifer's challenge wasn't just market competition or customer acquisition, but the intricate web of FDA regulations, clinical evidence requirements, and compliance documentation that governed every aspect of their go-to-market strategy. She explained how their launch timeline extended from 18 months to nearly three years due to regulatory requirements, but also how this thorough process ultimately created competitive advantages through demonstrated safety, efficacy, and regulatory compliance that competitors couldn't easily replicate. Jennifer's experience illustrated the unique dynamics of executing GTM strategies in regulated industries where compliance isn't just a requirement but a strategic differentiator.
Go-to-market strategies in regulated industries require fundamentally different approaches that balance aggressive growth objectives with strict compliance requirements. These strategies must navigate complex regulatory frameworks while building trust, demonstrating value, and achieving sustainable market penetration within constrained operational parameters.
Navigating Legal and Compliance Requirements
Regulatory compliance in modern GTM strategies extends beyond simple legal adherence to encompass proactive regulatory intelligence that informs strategic decision-making. Successful companies in regulated industries develop comprehensive regulatory monitoring systems that track pending legislation, enforcement trends, and regulatory guidance that could impact their market strategies.
The integration of legal expertise into GTM planning has become essential for regulated industries. Legal teams now participate in strategic planning sessions, product positioning discussions, and campaign development to ensure compliance considerations are incorporated from the beginning rather than addressed reactively. This approach prevents costly delays and reduces the risk of non-compliance issues.
Compliance documentation has evolved from static legal requirements to dynamic strategic assets that support market positioning and competitive differentiation. Companies now leverage their regulatory approvals, certifications, and compliance history as proof points that build customer confidence and justify premium pricing positions.
Digital compliance monitoring has transformed how regulated companies manage ongoing compliance requirements. Automated systems now track regulatory changes, monitor competitive compliance status, and alert marketing teams to potential compliance risks before they impact market activities. This proactive approach enables faster response to regulatory changes while maintaining continuous compliance.
The emergence of regulatory technology platforms enables more efficient compliance management across multiple jurisdictions and regulatory frameworks. These systems standardize compliance processes, automate documentation requirements, and provide audit trails that support both regulatory compliance and competitive intelligence activities.
Implementing Messaging Guardrails
Messaging development in regulated industries requires sophisticated frameworks that balance compelling value proposition communication with strict regulatory requirements. Modern messaging strategies use compliance-approved language libraries, pre-approved claim substantiation, and automated content review processes to ensure all customer communications meet regulatory standards.
The challenge of creating compelling messages within regulatory constraints has led to innovative approaches to value proposition development. Regulated companies now focus on outcome-based messaging, evidence-based claims, and customer success stories that demonstrate value while adhering to regulatory limitations on promotional claims.
Content governance systems have become essential for maintaining messaging consistency across multiple channels and touchpoints. These systems ensure that all marketing materials, sales presentations, and customer communications use approved language and substantiated claims while maintaining brand consistency and competitive positioning.
Digital messaging compliance has introduced new complexities as companies expand their digital marketing efforts. Social media guidelines, website content standards, and email marketing compliance requirements now require specialized expertise and automated monitoring systems to ensure ongoing compliance across all digital touchpoints.
The development of compliance-approved messaging frameworks enables marketing teams to create compelling content without extensive legal review for every piece. These frameworks provide approved language options, substantiated claim templates, and guidance for adapting messages to different audiences while maintaining regulatory compliance.
Managing Slower Launch Cycles
Extended launch timelines in regulated industries require sophisticated project management approaches that coordinate regulatory requirements with market opportunity windows. Successful companies use parallel processing techniques, conditional planning scenarios, and milestone-based resource allocation to optimize launch efficiency within regulatory constraints.
Competitive advantage strategies during extended launch cycles focus on building market relationships, establishing thought leadership, and preparing distribution channels before product availability. Companies use this extended timeline to educate markets, build anticipation, and establish competitive positioning that supports successful product launches when regulatory approval is achieved.
Stakeholder engagement during extended launch cycles has become crucial for maintaining momentum and support throughout lengthy regulatory processes. This includes ongoing communication with investors, customers, distribution partners, and internal teams to maintain confidence and commitment despite extended timelines.
Market readiness preparation during regulatory review periods enables companies to launch immediately upon approval rather than beginning market preparation after regulatory clearance. This approach includes sales team training, distribution channel development, and customer education initiatives that can be activated quickly when regulatory approval is received.
The integration of regulatory milestone tracking with market readiness planning enables companies to coordinate internal capabilities with external regulatory progress. This approach ensures that market launch capabilities are fully developed and ready for immediate activation when regulatory approval is achieved.
Case Study: Teladoc Health's Regulatory Navigation Strategy
Teladoc Health's expansion into regulated healthcare markets demonstrates sophisticated GTM strategy execution within complex regulatory environments. As telemedicine regulations varied significantly across states and specialties, Teladoc developed a comprehensive regulatory compliance framework that enabled market expansion while maintaining strict adherence to healthcare regulations.
Their approach to legal and compliance navigation included establishing dedicated regulatory affairs teams for each market segment, developing state-specific compliance protocols, and creating automated monitoring systems that tracked regulatory changes across all operating jurisdictions. This infrastructure enabled rapid adaptation to regulatory changes while maintaining continuous compliance.
Messaging guardrails included development of specialty-specific communication frameworks, physician education materials that met continuing education requirements, and patient communication protocols that complied with healthcare privacy regulations. Their messaging strategy balanced compelling value propositions with strict healthcare marketing limitations.
Managing slower launch cycles required coordinated planning across multiple regulatory approval processes, state-by-state market entry strategies, and phased rollout approaches that aligned with regulatory clearance timelines. Teladoc used extended preparation periods to build provider networks, educate target markets, and establish competitive positioning before full market entry.
Their regulatory compliance framework included real-time monitoring of healthcare regulations, automated compliance reporting systems, and proactive regulatory intelligence that informed strategic planning decisions. This approach enabled them to identify market opportunities while avoiding regulatory pitfalls that could delay market entry.
The results demonstrate the effectiveness of their regulatory-first GTM approach. Teladoc successfully expanded across multiple regulated markets while maintaining regulatory compliance and achieving sustainable growth. Their systematic approach to regulatory navigation has become a competitive advantage that enables faster market entry than competitors with less sophisticated regulatory capabilities.
Call to Action
Organizations operating in regulated industries should begin by conducting comprehensive regulatory landscape assessments that identify all applicable regulations, pending regulatory changes, and competitive regulatory positioning. Develop internal regulatory expertise or establish partnerships with specialized regulatory consultants who understand your industry's unique requirements.
Create integrated planning processes that coordinate regulatory compliance activities with marketing strategy development, ensuring that compliance considerations inform strategic decisions rather than constraining them retroactively. Invest in regulatory technology platforms that automate compliance monitoring, documentation, and reporting requirements while providing competitive intelligence capabilities.
Develop messaging frameworks and content governance systems that enable efficient marketing execution within regulatory constraints. Establish milestone-based planning processes that optimize resource allocation and market readiness preparation throughout extended regulatory approval cycles. Remember that regulatory compliance can become a competitive advantage when executed systematically and strategically.
Featured Blogs
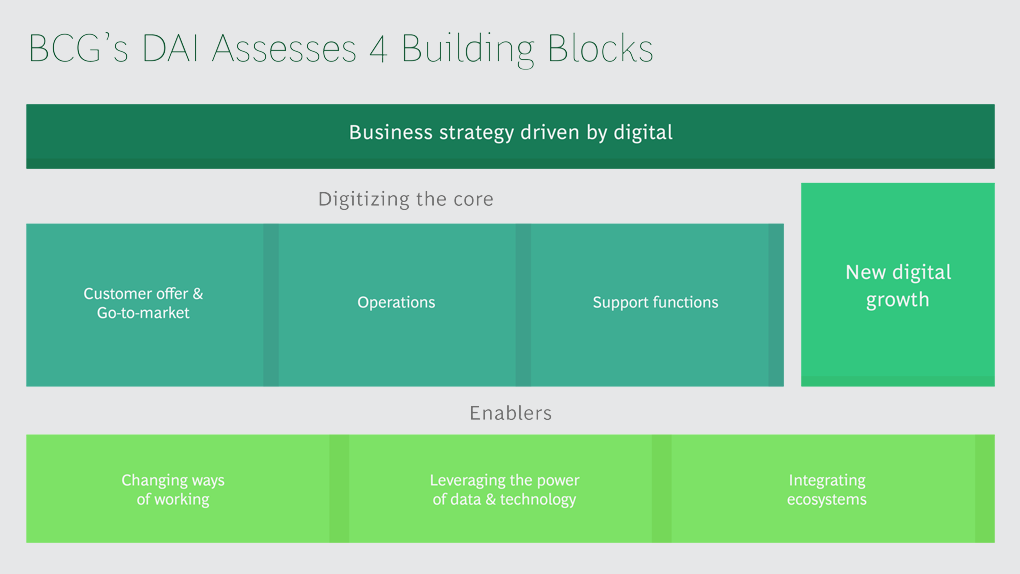
BCG Digital Acceleration Index
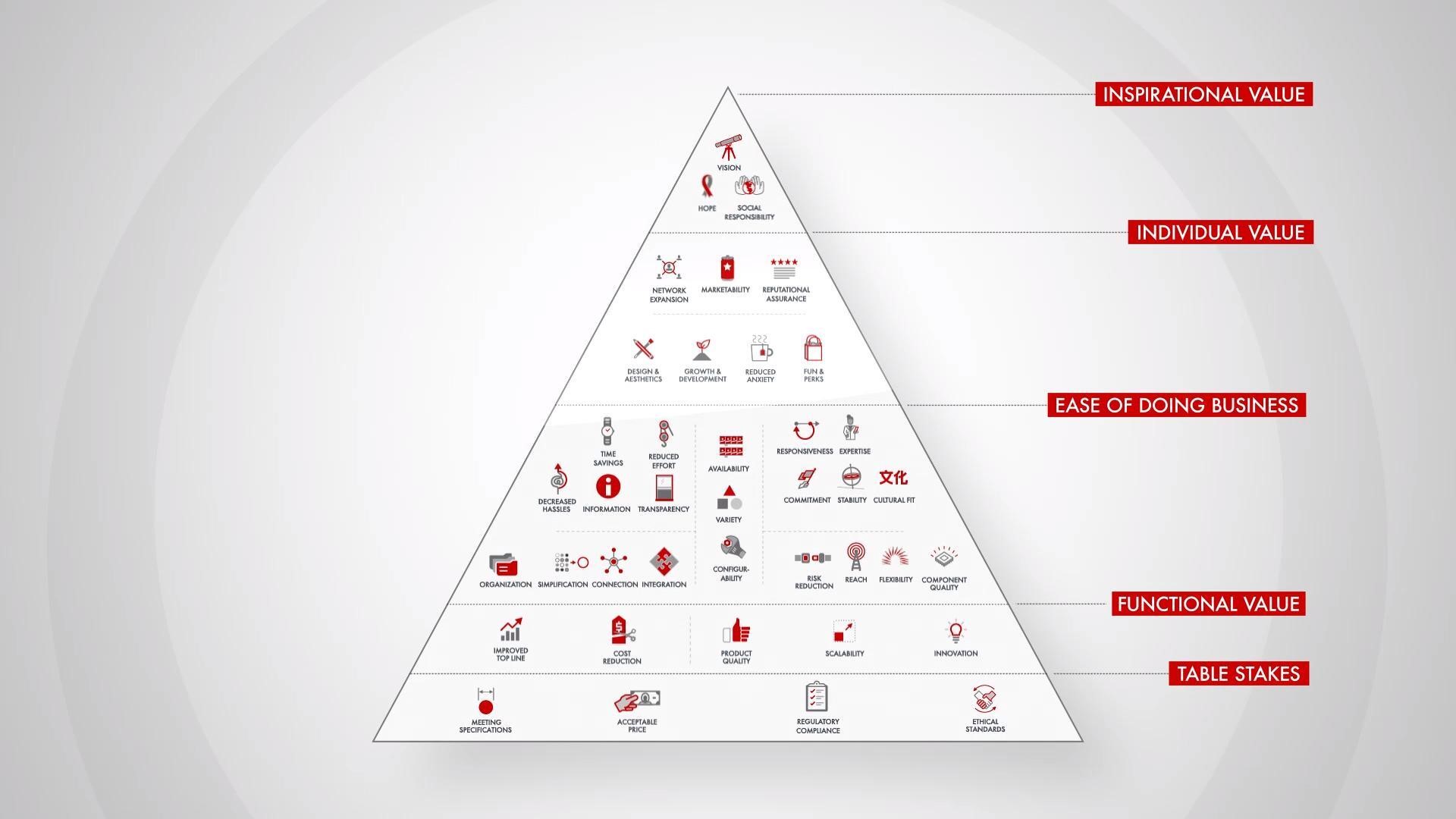
Bain’s Elements of Value Framework
McKinsey Growth Pyramid
McKinsey Digital Flywheel
McKinsey 9-Box Talent Matrix
McKinsey 7S Framework
The Psychology of Persuasion in Marketing

The Influence of Colors on Branding and Marketing Psychology