Regulatory Triggers for Global Media Campaign Management
Last week, I met with Jennifer, global marketing compliance director at a major pharmaceutical company, who shared her nightmare experience when their respiratory medication campaign was rejected in twelve countries simultaneously due to varying regulatory requirements they hadn't anticipated. What should have been a straightforward global launch became an eighteen-month compliance marathon that cost the company an estimated $40 million in delayed market entry and regulatory penalties. Her experience highlighted the critical importance of understanding regulatory triggers before launching global campaigns, particularly in highly regulated industries where compliance failures can devastate both timelines and profitability.
The complexity of global regulatory compliance has intensified dramatically as digital advertising transcends traditional geographic boundaries while regulatory frameworks remain distinctly national. With over 195 countries maintaining unique advertising regulations, and regulatory changes occurring monthly across major markets, the ability to navigate compliance requirements has become as crucial as creative excellence for successful global campaigns. Industries like pharmaceuticals, alcohol, financial services, and political advertising face particularly complex regulatory landscapes that can vary dramatically even between neighboring countries.
1. Understanding Country-Specific Advertising Guidelines and Compliance Frameworks
The foundation of effective global regulatory management lies in comprehensive understanding of country-specific advertising standards that extend far beyond simple content restrictions. Modern regulatory frameworks encompass disclosure requirements, demographic targeting limitations, platform-specific rules, and industry-specific compliance standards that create complex matrices of requirements across international markets.
Pharmaceutical advertising regulations exemplify this complexity, where some countries permit direct-to-consumer advertising with specific disclaimers while others prohibit any consumer-facing pharmaceutical promotion entirely. The European Union's strict pharmaceutical advertising directives contrast sharply with the United States' FDA regulations, while emerging markets often have evolving frameworks that change rapidly as regulatory authorities adapt to digital advertising innovations.
Financial services regulations present another layer of complexity where advertising claims about investment returns, insurance benefits, or lending terms must comply with local financial authority requirements. Countries like the UK require specific risk warnings and disclosure formats, while markets like Singapore have strict regulations about targeting criteria and demographic restrictions for financial advertising.
Political advertising regulations vary dramatically across jurisdictions, with some countries requiring extensive disclosure of funding sources, others prohibiting foreign political advertising entirely, and many implementing seasonal restrictions around election periods. The intersection of political advertising rules with social media targeting capabilities creates particularly complex compliance challenges for global campaigns.
The strategic approach to regulatory compliance involves developing comprehensive regulatory intelligence systems that track changes across target markets, maintain current compliance requirements, and provide early warning systems for regulatory changes that might affect campaign planning and execution timelines.
2. Strategic Clearance Timeline Planning for Multi-Market Campaign Coordination
Effective regulatory clearance planning requires sophisticated project management that accounts for varying approval timelines, revision processes, and regulatory authority response patterns across different markets. The coordination of clearance processes across multiple jurisdictions often determines whether global campaigns can launch simultaneously or require staggered rollouts that may affect marketing effectiveness.
Pharmaceutical campaigns typically require the longest clearance timelines, with some markets requiring 90-120 days for regulatory approval while others can process submissions within 30 days. The strategic challenge involves balancing speed-to-market objectives with thorough compliance review processes that prevent costly rejections and revision cycles.
Alcohol advertising clearance presents unique challenges where religious and cultural considerations intersect with regulatory requirements. Markets with Islamic legal frameworks often have complete prohibition on alcohol advertising, while others permit advertising with specific time restrictions, content limitations, or warning requirements that must be integrated into creative development.
The development of clearance timeline strategies requires detailed analysis of regulatory authority processes, typical review duration, common rejection reasons, and appeal procedures that may be necessary if initial submissions are denied. Successful global campaigns often build 6-12 month clearance buffers for complex regulatory environments.
Advanced clearance planning also involves developing regulatory relationship management strategies that facilitate faster processing through consistent compliance demonstration, proactive communication with regulatory authorities, and collaborative approaches that reduce adversarial dynamics between brands and regulators.
3. Industry-Specific Regulatory Navigation for Pharmaceutical, Political, and Alcohol Marketing
The development of industry-specific regulatory expertise represents a critical competitive advantage for brands operating in heavily regulated sectors. Each industry faces unique compliance challenges that require specialized knowledge, dedicated resources, and sophisticated management systems that can adapt to evolving regulatory landscapes.
Pharmaceutical regulatory navigation requires understanding of therapeutic area-specific restrictions, clinical trial disclosure requirements, adverse event reporting obligations, and healthcare professional targeting limitations that vary significantly across markets. The integration of digital advertising capabilities with traditional pharmaceutical compliance frameworks creates ongoing challenges that require continuous regulatory monitoring and adaptation.
Political advertising regulations have become increasingly complex as digital platforms implement their own compliance requirements in addition to government regulations. The intersection of platform policies, national election law, foreign interference restrictions, and transparency requirements creates multi-layered compliance challenges that require specialized expertise and sophisticated monitoring systems.
Alcohol advertising represents perhaps the most culturally sensitive regulatory environment, where religious considerations, public health policies, and social responsibility frameworks intersect with commercial advertising rights. Markets like India require state-by-state compliance assessment, while Middle Eastern markets often have complete prohibition that requires careful geo-targeting to avoid regulatory violations.
The strategic framework for industry-specific navigation involves developing specialized regulatory teams, maintaining relationships with local legal counsel across key markets, and implementing systematic compliance monitoring that tracks regulatory changes before they impact campaign execution. This proactive approach enables brands to adapt strategies before compliance issues become campaign-stopping problems.
Advanced industry-specific strategies also involve regulatory risk assessment that evaluates the potential impact of compliance failures, including financial penalties, market access restrictions, and reputational damage that can affect long-term business performance beyond immediate campaign results.
Case Study: Novartis Global Campaign Regulatory Transformation
Novartis faced a critical challenge when their traditional market-by-market regulatory approach resulted in inconsistent campaign launches, missed opportunities, and significant compliance costs across their international operations. Different regulatory timelines were creating 6-12 month delays between market launches, reducing campaign effectiveness and increasing competitive vulnerabilities.
The company implemented a comprehensive regulatory intelligence system that mapped compliance requirements across 65+ markets, identifying common approval pathways, typical processing timelines, and frequent rejection reasons that could be addressed proactively in campaign development. Their new framework treated regulatory compliance as a strategic advantage rather than a necessary burden.
Novartis developed market cluster strategies that grouped countries with similar regulatory frameworks, enabling shared compliance approaches that reduced duplicated effort while maintaining local compliance requirements. European Union markets were managed through centralized regulatory strategies, while emerging markets received specialized compliance support that addressed unique local requirements.
The company implemented sophisticated regulatory timeline modeling that enabled simultaneous campaign development and regulatory submission across multiple markets. Rather than sequential approvals, their new process involved parallel regulatory engagement that compressed overall clearance timelines by 60-70% across most markets.
Novartis also established regulatory relationship management programs that facilitated ongoing dialogue with key regulatory authorities, enabling proactive discussion of campaign concepts before formal submission. This collaborative approach reduced rejection rates by 45% while accelerating approval timelines through early identification and resolution of potential compliance issues.
Their technology infrastructure included automated regulatory change monitoring that tracked updates across all target markets, providing early warning systems for regulatory changes that might affect campaign strategies. This proactive intelligence enabled strategic adaptation before compliance issues became campaign obstacles.
Results demonstrated significant competitive advantages with 280% faster global campaign launches, 65% reduction in regulatory compliance costs, and 90% improvement in first-submission approval rates. Most importantly, their regulatory excellence enabled market entry advantages that generated an estimated $180 million in additional revenue through faster product launches and competitive positioning.
Conclusion
Regulatory triggers represent one of the most critical yet underestimated aspects of global marketing success. As digital advertising continues to transcend traditional boundaries while regulatory frameworks remain distinctly national, the ability to navigate complex compliance requirements will increasingly determine competitive advantage in international markets.
The future of global regulatory management lies in the integration of artificial intelligence with regulatory intelligence, creating systems that can predict regulatory changes, optimize compliance strategies, and enable proactive adaptation to evolving regulatory landscapes. Brands that invest in sophisticated regulatory capabilities now will establish sustainable competitive advantages that become increasingly valuable as global marketing complexity continues to evolve.
The transformation from reactive compliance to strategic regulatory management requires fundamental shifts in how global brands approach market entry, campaign development, and risk management. Organizations that successfully navigate this evolution will find themselves uniquely positioned to capitalize on global opportunities while avoiding regulatory pitfalls that can devastate unprepared competitors.
Call to Action
Global marketing leaders should conduct comprehensive regulatory audits of their current compliance capabilities, identifying gaps in regulatory intelligence, clearance timeline management, and industry-specific expertise. Invest in sophisticated regulatory monitoring systems, develop specialized compliance teams, and establish proactive relationships with regulatory authorities across key markets. The brands that build regulatory excellence into their global marketing strategies will establish competitive advantages that enable faster market entry, reduced compliance costs, and sustainable international growth in an increasingly complex regulatory environment.
Featured Blogs
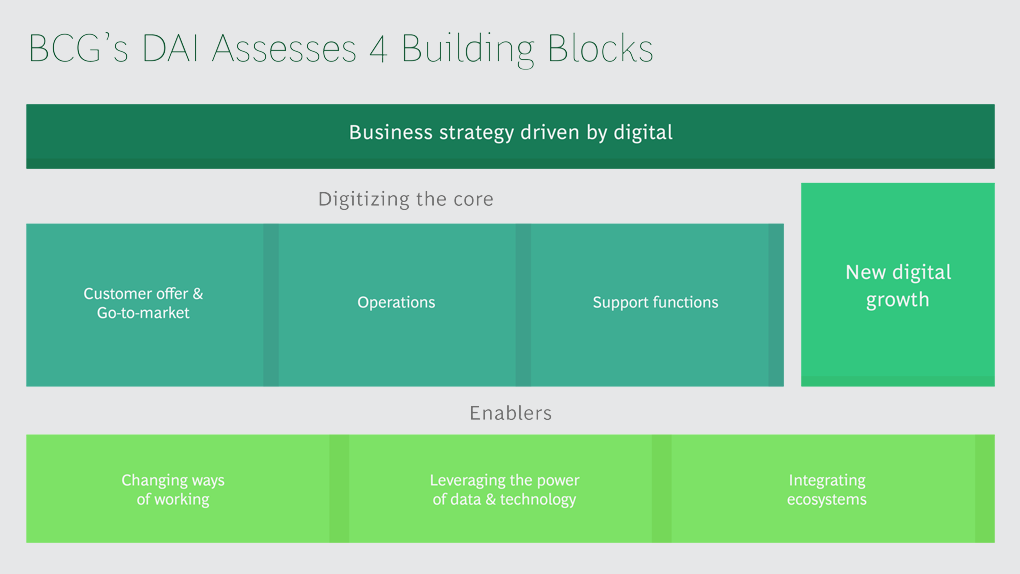
BCG Digital Acceleration Index
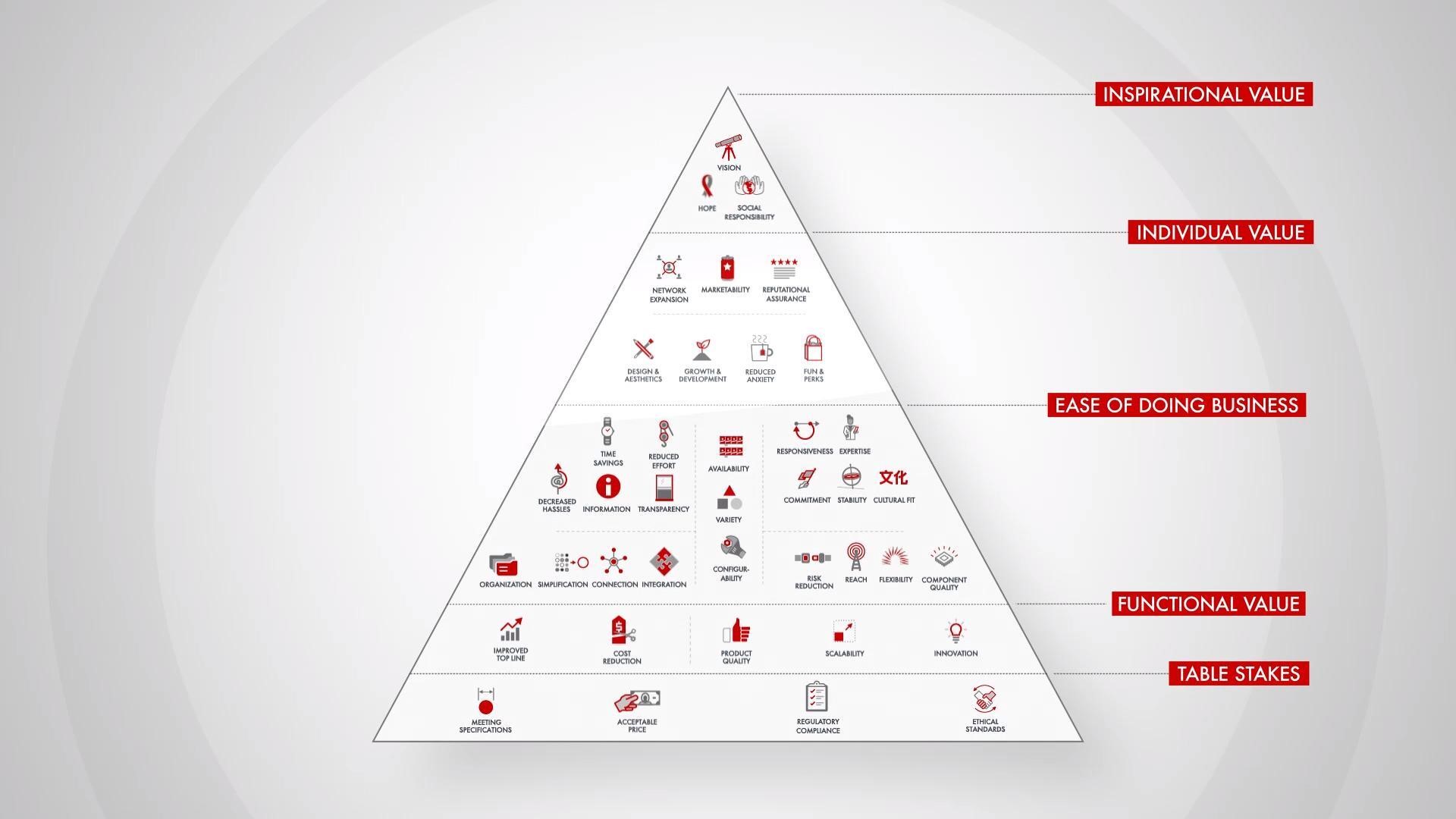
Bain’s Elements of Value Framework
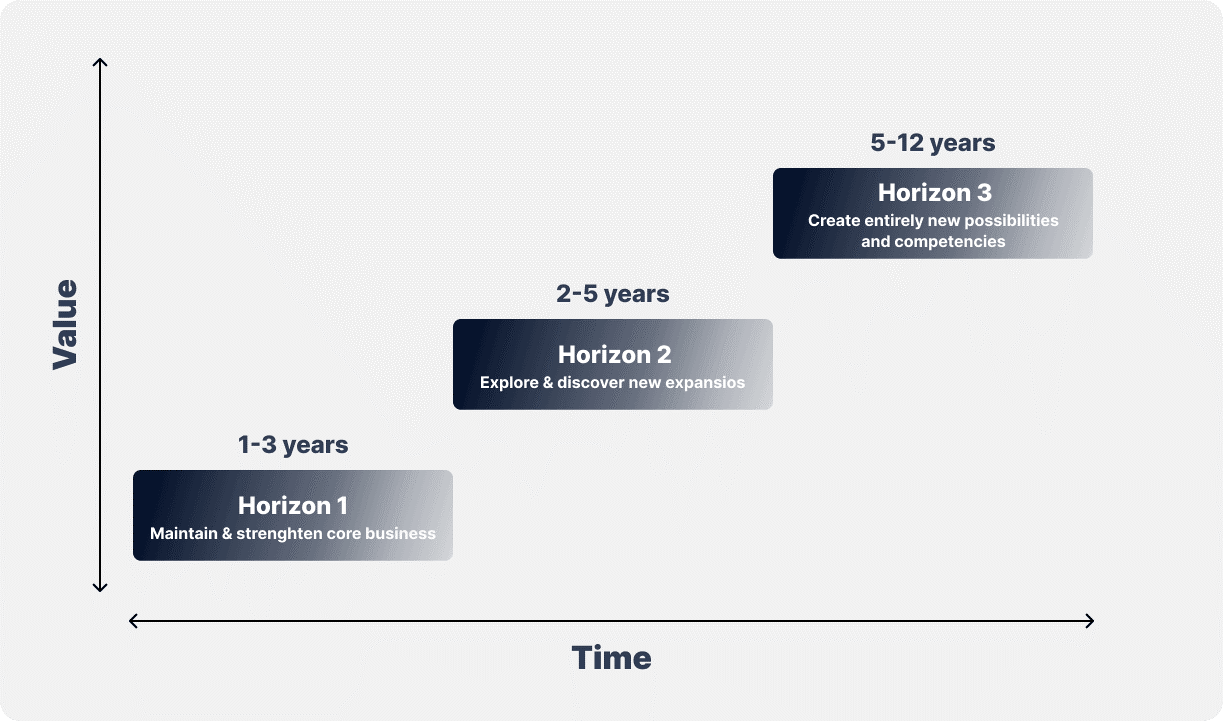
McKinsey Growth Pyramid
McKinsey Digital Flywheel
McKinsey 9-Box Talent Matrix
McKinsey 7S Framework
The Psychology of Persuasion in Marketing

The Influence of Colors on Branding and Marketing Psychology